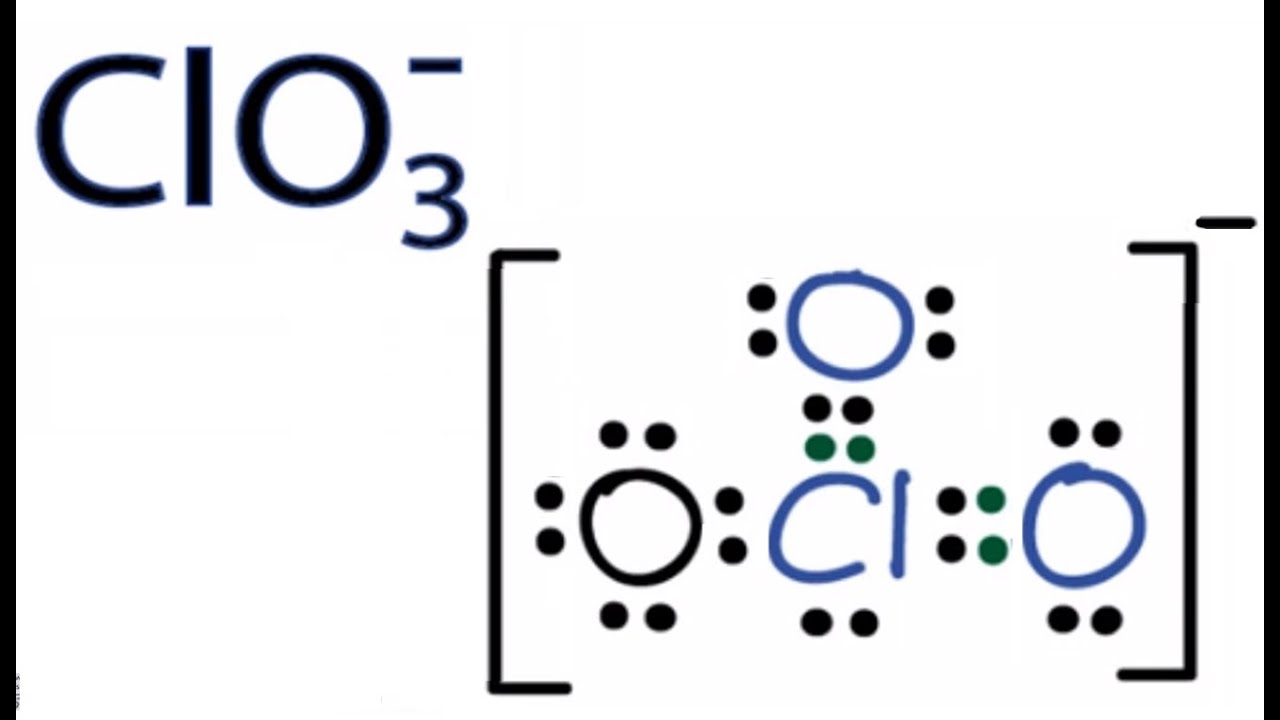Lewis Structure Of Cl3- Solved Draw The Best For Cl3" What Is The Formal
Here are the steps to draw the clf3 lewis structure: Determine its molecular geometry and the hybridization of the central cl atom. The chlorine atom (cl) is.
ClO3 Lewis Structure, Molecular Geometry, Hybridization, and Polarity
Count the total number of valence electrons in clf3 by adding the valence electrons of each atom. The molecular geometry of cl3+ is trigonal planar with bond angles of. Lewis structure for cl3⁻ the lewis structure for cl3⁻ is drawn by following these steps:
Draw a lewis structure for cl 3 +.
The lewis structure for cl3 is: Count the total number of valence electrons. Chlorine has 7 valence electrons, and. You might think you've got the correct lewis structure for.
The lewis structure for cl3 would show three chlorine atoms bonded together with single bonds, and each chlorine atom having three lone pairs of electrons. Chlorine has 7 valence electrons, and there. Draw the lewis structure, predict the molecular structure, and describe the bonding (in terms of the hybrid orbitals for the central atom) for xeo3. Draw and explain the lewis structure for.